Scientists at Tsinghua University, Beijing, and the Massachusetts Institute of Technology, Boston have been working on techniques to extend the working life of Li-ion batteries. The scientists were working methodically on making the anodes of the batteries more durable, as the decay of the anode material is a major cause of the drop off in Li-ion battery life after a few hundred charge cycles. In the course of their carefully timed experimental process one batch of anodes were left under chemical treatment for several hours more than they should have been, by accident. Instead of throwing away the forgotten batch, the scientists decided to test them and the results were great – an anode 4x more durable than the current best available technology.
Dr Wang Changan at Tsinghua and Dr Li Ju at MIT were behind the experiments. These materials scientists had been working on using aluminium for the anode of a Li-ion battery in preference to graphite. Today's Li-ion batteries use carbon anodes because aluminium expands and contracts too much during each charge/discharge cycle, so in effect self destructs pretty quickly.
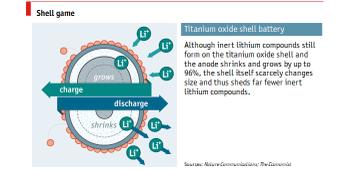
The scientists were working on stopping the oxide coating forming on aluminium nanoparticles when they are exposed to air by soaking the material in sulphuric acid and titanium oxysulphate. That replaces the aluminium oxide with a more conductive titanium oxide. As mentioned in the intro, timed soaking sessions were undertaken trying to get the timing right for creating the most durable anode.
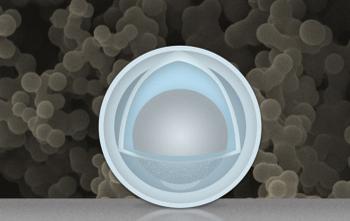
One errant batch got accidentally soaked and left in the chemicals for several hours longer than intended. Luckily the scientists decided to test these samples - as they found they had nearly all the advantageous properties they had been looking for in a new anode. The new anode formed with a solid nanoparticle shell and an inner 'yolk' of aluminium material that could expand and contract without damaging the coating.
Dr Wang and Dr Li created some batteries using the 'accidental anodes' with the nanoparticle coating and ran them through 500 charge/discharge cycles. Compared to graphite-electrode equivalents put through the same charging cycle these new batteries retained as much as four times the capacity. The manufacture of these nanoparticle imbued shell, plus aluminium yolk, anodes is reasonably likely to be commercialised as the process seems to be both simple and scalable.